
A creationist view of phylogenetic change in the equid family
Paul Garner BSc (Hons)
Introduction
The fossil record of horses has often featured in the debate between creationists and evolutionists about origins. Horses are particularly well represented in the geological record, especially in North America, and appear to form a convincing, though complex, evolutionary sequence. Many see the horse series as a major challenge to a creationist view of earth history. Thus, evolutionists have cited the series as important evidence in support of their theory, while creationists have attempted to debunk it as artificial and composed of non-equivalent parts. I believe that both these positions are mistaken. In this article, I want to explain why. However, I do not wish to be merely negative. The ethos of the Biblical Creation Society is to encourage the development of strong and vigorous alternatives to evolutionary thinking. In that spirit I will propose an alternative creationist approach to the famous horse series, one that has been hinted at by others previously.
Some preliminary clarifications about the definition of ‘evolution’
It would perhaps be helpful if I first defined some terms. There is a great deal of confusion in the creationist and evolutionist literature about what we mean by ‘evolution’. My view is that the terminology proposed by Brand (1997 pp.111-26) is useful in dispelling some of the confusion (figure one). I am aware that some creationists will disagree with me about the value of this terminology - perhaps this article will stimulate a more extended discussion in Origins - but it provides a platform for us to think the issues through. Brand defines microevolution as change within a species, speciation as the origin of new species, and macroevolution as the origin of new genera, families, orders, or phyla. Megaevolution, a subset of macroevolution, is the origin of new families, orders, or phyla. Brand argues that a biblical creationist can accept microevolution and even some macroevolution, at least to the development of new genera. He describes some of the scientific evidence that supports this view, and points out that there is nothing in the Bible to contradict this. However, he rejects the idea of megaevolution and proposes limits to the changes that evolution can produce - there appears, for instance, to be no known genetic process that can produce entirely new body plans (Brand 1997 pp.168-71). Therefore, when we examine the data relating to fossil horses, we need to determine what level of evolutionary change we are dealing with.
All the extinct and extant horses mentioned in this article belong to the family Equidae of the order Perissodactyla (Carroll 1988 p.644). Therefore, if the horse series from Hyracotherium to Equus is a genuine phylogeny (‘family tree’), we are dealing with biological variation within the taxonomic rank of family. This level of change would, using Brand’s terminology, be defined as macroevolution, but would not be considered an example of megaevolution. Since it is only megaevolution that appears to meet insuperable biblical and scientific barriers, we can approach the horse series with minds open to the possibility that these fossil animals really were all genetically related.
A brief description of the horse series
Many scientists regard the horse series as powerful evidence in favour of evolutionary theory. In his major textbook on vertebrate palaeontology, Carroll (1988 p.533) states:
“The extensive fossil record of the family Equidae provides an excellent example of long-term, large-scale evolutionary change. Changes in body size, skull proportions, dentition, limb structure, and relative brain size have all been thoroughly documented.”
The horse series frequently features in books and articles written by opponents of creationism (e.g., Futuyma 1995 pp.85-94; McGowan 1984 pp.142-8; Monroe 1985). We need to review the horse series to see why so many scientists believe it offers evolutionary theory such strong support. We will look first at the so-called main line of evolution from Hyracotherium to Equus, as it is generally portrayed in textbooks and museum exhibits. Later, I will develop the point that this lineage is not just an oversimplification of the available data, but so grossly oversimplified that it is actually misleading.
Hyracotherium to Epihippus
According to current thinking, the root of the family tree of the horse is to be found in a small creature called Hyracotherium, whose fossils are known from the Lower Eocene of North America and Europe. Hyracotherium was first described by the famous naturalist and anti-evolutionist Richard Owen based on specimens recovered in 1838 from the London Clay (Owen 1840). Specimens found in North America were called ‘Eohippus’ (‘dawn horse’), but subsequent studies led most palaeontologists to conclude that ‘Eohippus’ and Hyracotherium were one and the same genus (Forster-Cooper 1932; Simpson 1952). Under the rules of zoological nomenclature the earlier name had precedence, so the name ‘Eohippus’ was dropped, although it is occasionally still found, erroneously, in popular presentations of the horse series.
Hyracotherium was a small mammal with four toes on the front feet and three on the rear. It was a brachyodont (i.e., had low-crowned teeth), with four premolars and three molars in each jaw. Its characteristics are those of a forest-dwelling animal that browsed on foliage. The lineage in North America can be traced from Hyracotherium (Lower Eocene) to Orohippus (Middle Eocene) and Epihippus (Upper Eocene). The differences between these three genera are slight. Monroe (1985 p.10) says that the upper premolars of Orohippus are more molariform than in Hyracotherium. According to Kitts (1957, p.1), “Orohippus and Hyracotherium are very similar to each other in almost all known anatomical characters”. Barnhart notes that the remains of Orohippus, mainly consisting of teeth, are found in only two formations in one geographic area, the Bridger Basin and Uinta Basin of Wyoming. He suggests that it is “no more than an unrecognized variation of another genus” (Barnhart 1984 p.111). The differences between Orohippus and Epihippus also appear to be primarily related to dentition. Monroe (1985 p.10) says of Epihippus, “The upper and lower third and fourth premolars were molariform and the first lower premolar was single-rooted rather than double-rooted as in Orohippus and Hyracotherium.”
Mesohippus to Parahippus
Mesohippus, a sheep-sized Oligocene form, had only three toes on the forefoot. As in Hyracotherium , the teeth were brachyodont, but all except the first premolar were now molariform. Mesohippus appears to have become extinct by the middle Oligocene (MacFadden 1992 p.175), and is thought to have given rise to the characteristic Miocene genus Merychippus via Parahippus . It is in Parahippus that the trend toward hypsodonty (i.e., high-crowned teeth) and the addition of cement to the cheek teeth is first seen. Referring to Parahippus , Carroll says (1988 p.534):
“The teeth became high crowned to resist the greater wear of a diet of hard grasses. To accommodate the long roots of the cheek teeth (about 60 per cent their length in modern horses), the jaws and face became deepened and the tooth row was displaced anteriorly relative to the orbit and the jaw articulation. The zygomatic arch was strengthened by the completion of the postorbital bar. The high-crowned teeth were supported by a newly elaborated tissue, cement, which is soft but tough and serves to support the hard but brittle columns of enamel. The pattern of the molar and premolar lophs approached those of the modern horse. The muzzle was elongated to extend the reach of the enlarged incisors.”
Merychippus
The Miocene genus Merychippus also had three toes, but the central one apparently bore most of the weight (Thomason 1986). The structure of the foot suggests that a strong elastic ligament, like that of modern horses, passed behind this central toe. The teeth were hypsodont, coated in cement, and had a more complex chewing surface.
“These specializations of the dentition and limbs point to Merychippus as a rapidly running, grazing animal of the newly expanded North American prairies” (Carroll 1988 p.535).
Pliohippus to Equus
According to the evolutionary sequence, a branch from the Merychippus line led to Pliohippus , a late Miocene to Pliocene form. In Pliohippus the side toes became vestigial - though some species are now known to have been tridactyl (MacFadden 1992 p.255) - and hypsodonty was increased further. A descendant lineage of Pliohippus gave rise to the Pleistocene genus Equus , which rapidly spread to Europe, Asia, Africa, and South America. However, by the end of the Pleistocene the genus was extinct in the New World. Nine species survive to this day in the Old World - the wild Asian horse, four species of asses, and four zebras (Carroll 1988 p.535).
The ‘major trends’ of horse evolution
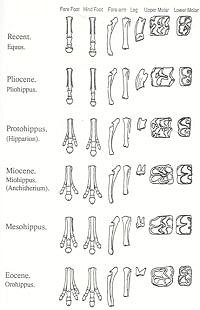
Figure Two
It is common to find this story presented in diagrammatic form illustrating what are considered to be the ‘major trends’ of horse evolution (increased body size, reduction of lateral toes, increased hypsodonty). One of the earliest such charts was prepared by the American vertebrate palaeontologist Othniel C. Marsh in 1876 for visiting lecturer Thomas Huxley (MacFadden 1992 pp.32-33). However, this diagram (figure two) was not drawn to scale, and therefore did not portray the marked size increase that would be made evident in subsequent presentations. A later example (figure three) produced in 1902 by palaeontologist William Matthew includes the trend towards increased body size (MacFadden 1992 p.45). Such diagrams are, as Gould (1997 p.57) points out, “the most pervasive of all evolutionary icons”. However, the issue we must address is the extent to which these diagrams reflect reality. Do these illustrations encapsulate real trends evident in the fossil record? Let us, then, examine the so-called ‘major trends’ of horse evolution.
In a limited sense, these trends are legitimate. There is a trend towards the loss of toes from four on the forefoot and three on the hindfoot of Hyracotherium (4/3), to 3/3 in Mesohippus , and 1/1 in Equus . There is a trend towards increased hypsodonty. All pre-Miocene horses were brachyodont, and crown heights increase from Merychippus to Equus . However, textbooks make these trends look much more gradual and unidirectional than they really are in the fossil record. As Gould (1997 p.61) has pointed out, Equus is the only living genus of horses and therefore the only modern animal that can serve as an endpoint for a series. This is in contrast to the multiplicity of contemporaneous genera and species revealed by the fossil record:
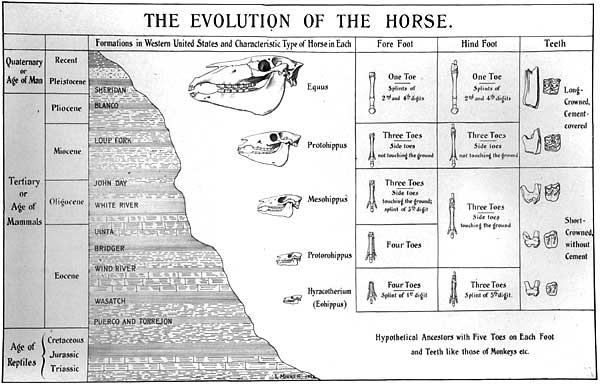
Figure Three
“The evolutionary bush of horses includes many terminal tips, and each leads back to Hyracotherium through a labyrinth of branching events. No route to Hyracotherium is straight, and none of the numerous labyrinthine paths has any special claim to centrality...We run a steamroller right over a fascinatingly complex terrain when we follow the iconographic convention for displaying the pathway from Hyracotherium to Equus as a straight line” (Gould 1997 p.63).
The straight-line, progressive change attributed to horses is therefore an artifact of their impoverished modern diversity. Furthermore, this phenomenon is not unique to horses:
“Many classic ‘trends’ of evolution are stories of such unsuccessful groups - trees pruned to single twigs, then falsely viewed as culminations rather than lingering vestiges of former robustness” (Gould 1997 p.64).
The increase in body size is one of the most striking features apparent in popular presentations of the horse sequence. However, MacFadden (1992 pp.216-22) provides an insight into the real complexities of the data. The increase in size between Hyracotherium and Equus was by no means gradual, constant, or progressive. An analysis by MacFadden (1987) of 24 ancestral-descendant species pairs revealed that 19 showed body-size increases. However, the remaining 5 lineages showed size decreases. There are even lineages within the horse family tree that show size decreases followed by reversals back to increased size (MacFadden 1984; Webb and Hulbert 1986; Hulbert 1988).
To drive home this point, Gould (1997 pp.70-1) raises the question of how we would view the fossil record of horses if the genus Nannippus had survived to the present-day instead of Equus . Bearing in mind the geographic distribution and longevity of Nannippus, Gould considers its survival a plausible, though unrealized, contingency. Nannippus was a dwarf horse, no higher than a Shetland pony and considerably more gracile. It was not much bigger than Hyracotherium , so there would be no evident progression towards increased body size. Nannippus even had three toes on each foot, so the tale about loss of side toes would also lose most of its force. In fact, according to Gould, “if Nannippus had survived and Equus died, we wouldn’t be telling any famous story about horses at all” (p.71).
MacFadden (1992 pp.216, 223) is obviously right to issue the following caution:
“Since the turn of the century, most of the foremost paleontologists involved in original research on fossil horses (e.g., Matthew, Stirton, and Simpson) have recognized that the gradual, progressive trends depicted for fossil Equidae are at best oversimplifications to illustrate general evolutionary patterns. The problem in the interpretation of these occurs when other scientists and the lay public, themselves far removed from the original data, seize the simplified essence of these general patterns, and consequently many of the details get lost in this process...Trends and directionality, though firmly entrenched in the literature and thought to be prime concepts within evolutionary theory, are, at best, broad patterns or generalizations.”
The real picture is more accurately portrayed in figure four, and even this oversimplifies the situation. This diagram shows the equid family tree constructed by Bruce MacFadden, the foremost modern authority on fossil horses (MacFadden 1992 p.99). Gould’s comment on this diagram is that it represents “only copious branching bushiness, and nothing that anyone could identify as a central thrust amid the diversity” (1997 p.65). Even the earlier “tolerably linear” part of the diagram is in reality a complex branching bush (Gould 1997 pp.67-9).
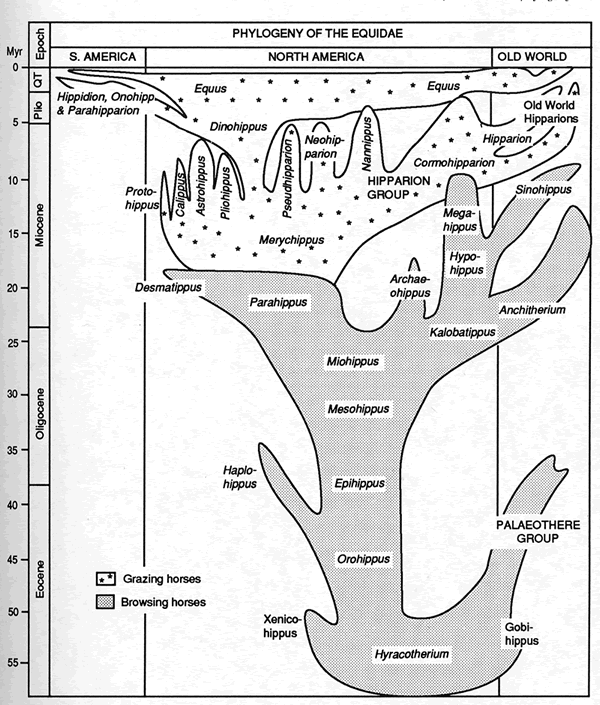
Figure Four
The fossil record of the horse as a stratomorphic series
Much of the debate between creationists and evolutionists about the fossil record centres around the existence, or otherwise, of ‘transitional forms’. In view of the interpretive and ambiguous nature of the term ‘transitional form’, Kurt Wise has proposed a new vocabulary which I believe is a helpful one (Wise 1995). For more details and discussion, the reader is directed to Wise’s paper on the subject. The three main new terms he has introduced are:
Stratigraphic intermediate fossil: a fossil which lies stratigraphically between two other fossils or between the lowest stratigraphic representatives of two fossil groups.
Morphological intermediate fossil: a fossil which is in some sense morphologically intermediate between two other fossils or between the shared characters of each of two other fossil groups. The type of morphological intermediacy (fully- versus partially-developed features, single versus multiple features, etc.) is not specified.
Stratomorphic intermediate fossil: a fossil which is both a stratigraphic intermediate and a morphological intermediate between two other fossils or two other fossil groups.
Using these, one can derive other terms, such as stratomorphic series. A stratomorphic series is a sequence of species or higher taxa where each taxon is a stratomorphic intermediate between the taxa stratigraphically below and above it. Bearing in mind all its complexity, the fossil record of the horse is an excellent example of a stratomorphic series. The question is, what are creationists to make of it?
Creationist apologetics and the horse series
Faced with the horse series as evidence for evolution, some creationists have argued that the series is artificial and does not exist in reality. In one sense, as we have seen, that is correct. Popular presentations usually suggest a simple, gradual, and progressive straight-line of evolution from Hyracotherium to Equus that is not supported by the actual fossil data. The real pattern is much more complicated and bush-like than the textbook diagrams and museum exhibits suggest. Evolutionary scientists readily acknowledge that this is the case. Niles Eldredge, for instance, commenting on an exhibit of fossil horses in the Hall of Tertiary mammals in the American Museum of Natural History, calls it “a classic of paleontologic museology” (Eldredge 1989 p.222). Futuyma (1995 p.90) says, “The history of horses, then, is very complex, and not at all the steady progress from Hyracotherium to the modern horse that is taught in introductory biology books”. None of this negates the series; it is merely that textbooks and museum displays are simplistic, portraying only selected trends. They do not reflect all the phylogenetic twists, turns, offshoots, and dead-ends that are evident in the fossil record.
However, I do not think that it is the complexity of the series that creationist authors have had in mind when they have argued that it is artificial. Take, for instance, this statement from Byron Nelson:
“The places in which they [i.e., fossil horses] are found are widely scattered, hundreds of miles apart, and the strata in which the fossils are found are surface strata. One fossil is not found below the other in any sense of the word. For all geological evidence there is to the contrary all the animals mentioned may have lived and died at the same time. Yet names are given to the rocks in which the fossils are found suggesting different ages (Eocene, Oligocene, Miocene, Pliocene, Pleistocene, Recent), and the fossils themselves are arranged in a series from the smallest, Eohippus, a four-toed creature about the size of a fox, up to the largest, the modern single-toed horse, and people are told that this is the time order in which they lived, and that this is the line of ancestry of the horse.” (Nelson 1967 p.73)
In other words, all the different types of fossil horses lived and died at the same time, and are not actually buried in succession in the fossil record. They have been artificially arranged into a succession by scientists with a preconceived bias about the evolution of the horse. This view denies that there is a genuine stratigraphic sequence, let alone a genuine stratomorphic sequence. Can such a view be sustained? Is the geological succession from Eocene to Recent really so arbitrary?
The fact that you cannot find a complete sequence of horse fossils one above the other in a single location does not disprove the sequence. At any one locality on the earth’s surface only a small portion of the rock record is usually exposed, but geologists are able to reconstruct the complete sequence by using the well established principles of superposition, correlation, and the lateral tracing of rock layers into other areas (see Robinson 1997 for more information on how the geological sequence is pieced together). Furthermore, folding, faulting and erosion lead to strata of all ages being exposed at the Earth’s surface (figure five) so the fact that the different types of fossil horses are all found at the surface does not pose a problem or a contradiction to the sequence. Indeed, how else would palaeontologists have been able to excavate, reconstruct, and study a fossil horse unless its skeletal remains had been exposed at the surface and were thus accessible to them?
Other creationists have argued that the series is artificial because there are genetic discontinuities between the animals included in it (i.e., the series is made up of animals some of which are completely unrelated to one another). Take, for instance, the view expressed by Frank Cousins:
“The family tree of the horse is beautiful and continuous only in the textbooks. In the reality provided by the results of research it is put together from three parts, of which only the last can be described as including horses...The construction of the whole Cenezoic [sic] family tree of the horse is therefore a very artificial one, since it is put together from non-equivalent parts, and cannot therefore be a continuous transformation series.” (1971 p.83)
In his paper, Cousins argues that the three unrelated parts are as follows: (i) Hyracotherium and the other Eocene forms, (ii) Mesohippus-Parahippus, and (iii) all later forms. He considers that there are unbridged gaps between these three parts of the series and that only the last group consists of real horses. However, Cousin’s analysis has been critiqued by Monroe (1985). Let us look at the reasons why Cousins divides the fossil equids as he does. The division between the Eocene forms and Mesohippus appears to have been made on the basis of size and numbers of toes on the forefeet:
“Epihippus is the last of the old horses, while Mesohippus is the first of the new horses. Between these we have a very considerable jump. For the first horses were small animals, only as big as foxes, with four-toed forefeet; only with the latter did the large, three-toed type first occur.” (p.79)
It is true that Mesohippus was typically a little larger than the earlier Eocene forms, but we should be careful not to exaggerate the difference, particularly when we take into account the range in size between species within the same genus. Perhaps the more important point is the number of toes on the forefeet. Most Mesohippus specimens have three-toed forefeet, in contrast to the four toes of the Eocene equids. However, Monroe points out that, although not widely known, at least two Mesohippus individuals from the early Oligocene of Wyoming have been found with four toes on the front feet (p.14). This means that the loss of the extra toe occurred after the transition to Mesohippus, and that the number of toes cannot be used to draw a distinction between the Eocene equids and Mesohippus.
The second discontinuity, according to Cousins, is between Parahippus and all later horses (such as Merychippus). He writes:
“Thereafter the real horse, the new horse, first appeared. The breaking of an hypothetical evolutionary series can hardly be more definite than with the appearance of this type. One-toedness dominated, although quite clear rudiments of two side-toes may occur. But an important deviant type occurred with respect to the teeth and the nature of the dentition. The teeth of the horse are very high, prismatic, not rooted (enamel-folded), and richly covered in cement. In this respect they are structures unique in the whole fauna. Animals with teeth first occur in the upper Miocene. These ‘hypsodental [sic] ungulates’ appear all at once, without intermediate states. They are even naturally variable, just like other groups, since they at once appeared in full bloom. With Merychippus and Hipparion there is a rich group of Equus -like forms which are all separated from the former ‘brachyodontal’ groups by a gaping evolutionary gap.” (p.82)
Cousins appears to have included Merychippus in the “new horse” group because its dentition was more similar to those forms. However, as Monroe (p.14) points out, Merychippus was more like the “old horses” in that it was three-toed. The explanation for horses like Merychippus that have a mixture of “primitive” and “advanced” traits is that the changes in toes and teeth were not proceeding at the same rate. Cousins’ “gaping evolutionary gap” between Parahippus and later horses is artificial. Even the trend towards hypsodonty began in Parahippus. Stirton (1940 p.178) comments that the earliest species of Merychippus and the latest species of Parahippus were “hardly distinguishable”. Simpson (1953 p.104) draws attention to fossil populations that are “perfectly intermediate between Parahippus and Merychippus and so varying in the ‘diagnostic’ characters that assignment of individuals in a single population could be made to both genera and assignment of the population to one or the other is completely arbitrary”.
In summary, it is difficult to maintain the divisions made in Cousins’ analysis of fossil horses.
Other critics have pointed to the co-existence of different horse genera in the same sediments as evidence against the idea that these forms might be associated in any kind of ancestor-descendant relationship. For instance:
“In the Rattlesnake Formation of the John Day Country of northeastern Oregon, the three-toed Neohipparion is found with the one-toed horse, Pliohippus. No transitional forms between the two are found. In other cases ‘primitive’ species of a genus, such as those of Merychippus, are found in geological formations supposedly younger than those containing ‘advanced’ species.” (Gish 1995 p.193)
Gish (p.194) goes on to quote from MacFadden (1992, p.255) about the discovery of an “exquisitely preserved” population of Dinohippus from Ashfall Fossil Beds in northeastern Nebraska in which some individuals were three-toed and others were one-toed, and also MacFadden’s statement (pp.257-8) that in the mid to upper Miocene we find three co-existing groups of fossil horses with “diverse postcranial morphologies”: those with a “primitive” three-toed foot, those with a “advanced” three-toed foot, and those with just one toe. His conclusion is that the three-toed forms did not evolve into the one-toed forms, but that they were unrelated types living at the same time. However, is this conclusion really warranted? Does not the fact that within a population of Dinohippus - in other words, within a single genus - we have both one-toed and three-toed forms, surely imply that these forms are genetically related? Furthermore, it does not logically follow that because three-toed and one-toed forms overlap in time, one did not give rise to the other. We do not conclude from the fact that a mother’s lifespan overlaps with that of her offspring that she cannot be their mother. Similarly, there is nothing to prevent an ancestral population overlapping in time with a descendant population. What about the claim that no transitional forms exist between the three-toed and one-toed forms? If one gave rise to the other, would we not expect to find fossil evidence of the transition? That would depend on the nature of the transition. If, as I suggest later, the transition was accomplished by differential gene expression (i.e., the genetic information for side toes being switched off), then no transitional forms showing gradual reduction of side toes would be expected.
Another creationist author argues for separate ancestry of Hyracotherium, Orohippus, and Pliohippus based on the different numbers of rib pairs in these species. Hyracotherium has 18 pairs, Orohippus has 15 pairs, Pliohippus has up to 19 pairs, and Equus scotti has 18 pairs. He concludes, “The rib count denies any continuous evolution here” (Hiebert 1979 p.61). However, as Monroe (1985 p.16) points out, in mammals ribs are found on the thoracic vertebrae, and the number of thoracic vertebrae and hence the number of ribs varies. Within a single species the rib count is usually consistent, but this is not always the case. Modern horses may have 17, 18 or 19 pairs of ribs (Epstein 1971 p.422). Even in human beings, the number of rib pairs can vary (Pick and Howden 1977 p.128). Hence, on the basis of rib counts it is erroneous to draw the conclusion that the fossil genera mentioned could not have been genetically related.
An alternative creationist approach
The horse series must be considered in its broader geological context. It is one of several impressive mammalian stratomorphic series that have been documented in the Cenozoic, including camels, elephants, rhinoceroses, and titanotheres (Carroll 1988). It is surely significant, as Wise (1994 p.163) has pointed out, that almost all the stratomorphic series in the fossil record are found in the Cenozoic (with some, such as the mammal-like reptiles, in the Mesozoic). How the believer in a global Flood interprets these series will depend on the particular Flood model he or she adopts (Tyler 1997). If the traditional Whitcomb-Morris approach is taken, with the Flood ending very late in the Cenozoic, all these series need to be explained in terms of Flood-related events. Anti-creationist Kenneth Miller lampooned this approach in the following words:
“...the illusion of an evolutionary series was created by a differential sorting action which accompanied the flood. More bluntly put, Equus (the modern horse) tread water for a longer time than Mesohyppus [sic] (an intermediate form), and Hyracotherium, a small ancestral form, was a poorer swimmer still. Therefore, they have been buried in the imaginary ‘sequence’ from which we now dig them up. This incredible mechanism must account not merely for the horse series, but for every evolutionary sequence known!” (Miller 1982 p.87)
Without doubt this is a ridiculous caricature of the Whitcomb-Morris position, but there is an important point to be made here. How can we account for the formation of a plethora of well-documented vertebrate stratomorphic series during the Flood? No detailed explanation has yet been developed by advocates of the Whitcomb-Morris model. Other creationists place the Flood/post-Flood boundary lower in the stratigraphic record. Those who favour an end-Cretaceous boundary are able to interpret the Cenozoic mammal successions as genuine phylogenies but must explain the Mesozoic reptile successions in terms of Flood depositional processes, whereas those who favour an end-Carboniferous (or even lower) boundary are free to interpret the Mesozoic and Cenozoic vertebrate successions as genuine phylogenies.
In my view, fossil horses form a convincing within-family stratomorphic series, and one that is probably post-Flood. I agree with those creationists who have concluded that the series represents a real phylogenetic sequence (e.g., Wise 1995). If this is the case, what were the natural selective pressures driving the diversification that we can trace in the fossil record? Whether the Flood/post-Flood boundary is at the end of the Cretaceous or earlier in the geological record, the explanation in terms of selective pressures is likely to be similar. I mentioned earlier that the horse series must be viewed in context as one of several Cenozoic stratomorphic series among the mammals. Wise (1995 p.220) has pointed out that almost all of these involve similar morphological changes that reflect a drying and cooling continental climate, along with evidence from the fossil flora of a transition from woodland to grassland communities. For instance, there is a trend from browsing to grazing and increased hypsodonty in the herbivores, and a move from woodland to open land among the primates (Wise 1994 p.163). Creationists are able to explain the woodland-grassland change as a result of the drying and cooling of the post-Flood earth. Heat from subterranean sources and from cooling of new oceanic lithosphere during and after the Flood would have contributed to the accumulation of heat by the early post-Flood oceans. As the oceans cooled - a trend confirmed by oxygen isotope data from Cenozoic foraminifera (Vardiman 1996) - so would the continents, and floral and faunal communities would be expected to reflect this. The trend towards increased body size may also reflect global cooling (Stanley 1973). Wise (1995 p.221) comments that:
“Conventional theory has no explanation for the secular decrease in ocean temperature over this period, nor for the increase in grassland over this period (except for the ad hoc suggestion that the grasses must have evolved). Then conventional theory must suggest that high selection pressures caused parallel and convergent evolution to occur within a number of groups. Given the absence of a mechanism for the cooling and drying of the earth and the difficulty in independent creation of new genetic material in a number of groups, conventional theory is much less successful at explaining some of their favourite fossil evidence (namely the horse series) than is the creation model.”
How might the morphological changes documented in the horse series have come about in a creationist scenario? Creationism is often portrayed as a belief in biological fixity, which in a sense it is at higher taxonomic levels. However, at lower taxonomic levels the creationist who believes in a recent global Flood must account for large amounts of morphological change in a very short time scale. Are there biological mechanisms that would allow for rapid change? This question is addressed in some detail by Leonard Brand, and his new book is recommended reading for anyone interested in pursuing this subject further (Brand 1997). A major part of a creationist biological theory is that organisms were originally created with latent (i.e., phenotypically unexpressed) genetic information that gave them tremendous potential for within-kind diversification. One way in which this latent genetic potential is regulated is by differential gene expression. By this we mean that in living organisms there are mechanisms by which genes can be turned on (i.e., expressed) or turned off (i.e., not expressed). Brand asks, “...could horses have a regulatory ‘switch’ that determines whether their legs develop with one prominent toe with its single hoof, or whether three toes develop approximately equally? If so, and if other regulatory genes control size, teeth morphology, etc., then the evolution of horses may have been a fairly simple genetic process” (p.202).
Indeed, there is evidence for differential gene expression in modern horses. McGowan (1984 p.147) describes the work of Ewart (1894), who studied horse embryos and found that at an early stage of development tiny limb buds appeared beneath the splint bones. When he dissected these buds to determine their internal structure he found that they resembled toes, with caps at the end that he believed represented hooves. At the most advanced stage it was even possible to distinguish three individual elements in the buds, corresponding to the three bones in the side toes of Merychippus. After this, and prior to birth, the limb buds were lost. It appears that modern horses retain the genetic potential for extra toes, but that regulatory genes switch off the structural genes for side toes during embryological development. Occasionally, something goes awry with this regulatory mechanism and foals are born with side toes (e.g., Marsh 1879, 1892; Struthers 1893). Evidently, even small changes in regulatory genes can produce relatively large phenotypic effects. It is likely that the splint bones of modern horses are true vestigial structures (i.e., they testify to real morphological changes in history). That splint bones are vestigial is no less true because they can be shown to serve a valuable function today (Bergman and Howe 1990; Murris 1992). In modern evolutionary theory an organ does not need to be functionless to be considered vestigial. Its form or function may simply be reduced or altered in some way (Brand 1997 p.152).
Suggestions for future research
The creationist scenario I have outlined above is a starting point, not an end point. It suggests a host of possible research projects for creationist biologists. Based on current evidence, I have tentatively assumed that the boundary of the biblical ‘kind’ or baramin is at the family level in equids. However, confirmation of this awaits application of the methods of discontinuity systematics (ReMine 1990) or baraminology (Wise 1990) to the horse family. An issue that needs further discussion is whether Hyracotherium should be included in the equid baramin. Does Hyracotherium belong at the base of the horse phylogeny, any more than at the base of the phylogenies of the brontotheres, the rhinoceroses, the tapirs, or the chalicotheres? More research on the mechanisms that led to rapid post-Flood speciation within the horses and other Cenozoic mammals is needed. Which morphological changes depended upon regulatory genes acting on latent genetic information? What role did environmental stimuli play? What was the time scale over which the changes took place? There are many unanswered questions. If able creationists take up the challenge of answering them, it will give the lie to our opponents’ claim that creationism stifles genuine research and it will be another clear demonstration that it is possible to be a good scientist and a creationist.
Conclusion
Palaeontological and embryological data indicate that the horse series is a genuine phylogeny, but it does not constitute an example of megaevolution since the morphological change documented is within the taxonomic rank of family. It is possible for creationists to interpret this morphological change as within-kind diversification after the Flood. Since the magnitude and type of change represented by the horse series can be accommodated by both evolutionist and creationist models it cannot, therefore, distinguish between them. At best, in terms of the origins debate, the horse series is neutral data.
Acknowledgements
I would like to thank Bill Hoesch of the Institute for Creation Research for kindly supplying me with a copy of Walter Barnhart’s unpublished Master’s thesis on fossil horses, and Drs David Tyler and Nancy Darrall for their insightful comments and suggestions on an earlier draft of this article.
References
Barnhart, W.R. 1987. A critical evaluation of the phylogeny of the horse. Master of Science Thesis, Institute for Creation Research, Santee, 254pp.
Bergman, J. and G. Howe. 1990. “Vestigial organs” are fully functional. Creation Research Society Books, Terre Haute.
Brand, L. 1997. Faith, reason, and earth history. A paradigm of earth and biological origins by intelligent design. Andrews University Press, Berrien Springs, Michigan.
Carroll, R.L. 1988. Vertebrate paleontology and evolution. W.H. Freeman, New York.
Cousins, F.W. 1971. The alleged evolution of the horse, in: Patten, D.W., editor. A symposium on creation III. Baker Book House, Grand Rapids:67-85.
Eldredge, N. 1989. Life pulse. Episodes from the story of the fossil record. Penguin, London.
Epstein, H. 1971. The origin of the domestic animals of Africa. Africana Publishing Company, New York.
Ewart, J.C. 1894. The development of the skeleton of the limbs of the horse, with observations on polydactyly. Journal of Anatomy and Physiology 28:342-69.
Forster-Cooper, C. 1932. The genus Hyracotherium . A revision and description of new specimens found in England. Philosophical Transactions of the Royal Society of London Series B 221:431-48.
Futuyma, D.J. 1995. Science on trial. The case for evolution. Sinauer Associates, Sunderland, Massachusetts.
Gish, D.T. 1995. Evolution: the fossils still say NO! Institute for Creation Research, El Cajon.
Gould, S.J. 1997. Life’s grandeur. Vintage, London.
Hiebert, H. 1979. Evolution: its collapse in view? Horizon House, Beaverlodge, Canada.
Hulbert, R.C. 1988. Calippus and Protohippus (Mammalia, Perissodactyla, Equidae) from the Miocene (Barstovian-early Hemphillian) of the Gulf Coastal Plain. Bulletin of the Florida State Museum of Biological Sciences 32:221-340.
Kitts, D.B. 1957. A revision of the genus Orohippus (Perissodactyla, Equidae). American Museum Novitates (1864):1-40.
MacFadden, B.J. 1984. Systematics and phylogeny of Hipparion, Neohipparion, Nannippus, and Cormohipparion (Mammalia, Equidae) from the Miocene and Pliocene of the New World. Bulletin of the American Museum of Natural History 179:1-196.
MacFadden, B.J. 1987. Fossil horses from ‘Eohippus’ (Hyracotherium) to Equus: scaling, Cope’s law, and the evolution of body size. Paleobiology 12:355-69.
MacFadden, B.J. 1992. Fossil horses: systematics, paleobiology, and evolution of the family Equidae. Cambridge University Press, Cambridge.
Marsh, O.C. 1879. Polydactyle horses, recent and extinct. American Journal of Science 17:499-505.
Marsh, O.C. 1892. Recent polydactyle horses. American Journal of Science 43:339-55.
McGowan, C. 1984. In the beginning...a scientist shows why the creationists are wrong. Prometheus Books, Buffalo.
Miller, K.R. 1982. Special creation and the fossil record: the central fallacy. The American Biology Teacher 44:85-9.
Monroe, J.S. 1985. Basic created kinds and the fossil record of perissodactyls. Creation/Evolution (XVI):4-30.
Murris, H.R. 1992. Vestigial organs. A creationist re-investigation. Origins 5(13):10-6.
Nelson, B. 1967. After its kind. Bethany House, Minneapolis.
Owen, R. 1840. Description of the fossil remains of a mammal, a bird, and a serpent, from the London Clay. Proceedings of the Geological Society of London 3:162-6.
Pick, T.P and R. Howden, editors. 1977. Gray’s anatomy. Bounty Books, New York.
ReMine, W.J. 1990. Discontinuity systematics: a new methodology of biosystematics relevant to the creation model, in: Walsh, R.E. and C.L. Brooks, editors. Proceedings of the Second International Conference on Creationism, Volume II: Technical Symposium Sessions and Additional Topics. Creation Science Fellowship, Pittsburgh:207-16.
Robinson, S.J. 1997. The geological column: a concept foundational to flood geology. Origins (23):14-30.
Simpson, G.G. 1952. Notes on British hyracotheres. Zoological Journal of the Linnean Society of London 42:195-206.
Simpson, G.G. 1953. The major features of evolution. Simon and Schuster, New York.
Stanley, S.M. 1973. An explanation for Cope’s Rule. Evolution 27:1-26.
Stirton, R.A. 1940. Phylogeny of North American Equidae. University of California Publications Bulletin Department of Geological Sciences 25:165-198.
Struthers, J. 1893. On the development of the bones of the foot of the horse, and of digital bones generally and on a case of polydactyly in the horse. Journal of Anatomy and Physiology 28:51-62.
Thomason, J.J. 1986. The functional morphology of the manus in the tridactyl equids Merychippus and Mesohippus : paleontological inferences from neontological models. Journal of Vertebrate Paleontology 6:143-61.
Tyler, D.J. 1997. Flood models and trends in creationist thinking. Creation Matters 2(3):1-3.
Vardiman, L. 1996. Cooling of the ocean after the Flood. Institute for Creation Research Impact Article #277.
Webb, S.D. and R.C. Hulbert. 1986. Systematics and evolution of Pseudhipparion (Mammalia, Equidae) from the late Neogene of the Gulf Coastal Plain and the Great Plains, in: Flanagan, K.M. and J.A. Lillegraven, editors. Vertebrates, phylogeny, and philosophy. Contributions to Geology, University of Wyoming, Special Paper 3.
Wise, K.P. 1990. Baraminology: a young-earth creation biosystematic method, in: Walsh, R.E. and C.L. Brooks, editors. Proceedings of the Second International Conference on Creationism, Volume II: Technical Symposium Sessions and Additional Topics. Creation Science Fellowship, Pittsburgh:345-60.
Wise, K.P. 1994. Australopithecus ramidus and the fossil record. Creation
Ex Nihilo Technical Journal
8:160-5.
Wise, K.P. 1995. Towards a creationist understanding of ‘transitional forms’. Creation Ex Nihilo Technical Journal 9:216-22.
FOOTNOTE (added September 2004):
The main conclusion of this article that the horse series represents rapid, within kind diversification after the Flood has since been confirmed by further work. At the 2003 International Conference on Creationism, a team from the Baraminology Study Group presented an analysis of nineteen fossil horse species in which they also concluded that the horse series was real:
Cavanaugh, D.P., Wood, T.C., Wise, K.P. Fossil Equidae: a monobaraminic, stratomorphic series, pp.143 153 in Ivey, R.L. ed. Proceedings of the Fifth International Conference on Creationism. Creation Science Fellowship, Pittsburgh, 2003.
In 2004, Paul Garner developed the work further in a paper given to the 'Discovering the Creator' conference at Bryan College, Dayton, Tennessee. This paper focused on the base of the horse series and the status of Hyracotherium . The abstract of this paper is available in the conference proceedings which can be downloaded here: http://www.bryancore.org/bsg/opbsg/004.html
This article first appeared in Origins, No 25, October 1998.

